All products featured are independently chosen by us. However, SoundGuys may receive a commission on orders placed through its retail links. See our ethics statement.
Where do batteries come from? And where do they go?
March 20, 2025
Every day, you use some type of battery. Your phone runs on a rechargeable lithium-ion battery, as do most of your other electronic devices. Your computer’s motherboard contains a non-rechargeable lithium coin cell, known as CMOS battery. Your car’s combustion engine starts on a rechargeable wet cell battery, typically the lead acid type. The list goes on.
This battery article was updated on:
- March 20, 2025: to identify the headphones with the longest battery life.
- March 7, 2023: to address a reader-submitted question.
Batteries have a limited lifespan. AirPods batteries will last anywhere from 18 months to three years. In 2021, around 300 million true wireless earbuds (TWS) were sold globally, and experts expect the market to grow further. As a result, we can expect over 450 million of those batteries to reach their end-of-life by the end of 2023 and more thereafter. And that’s just earbuds.
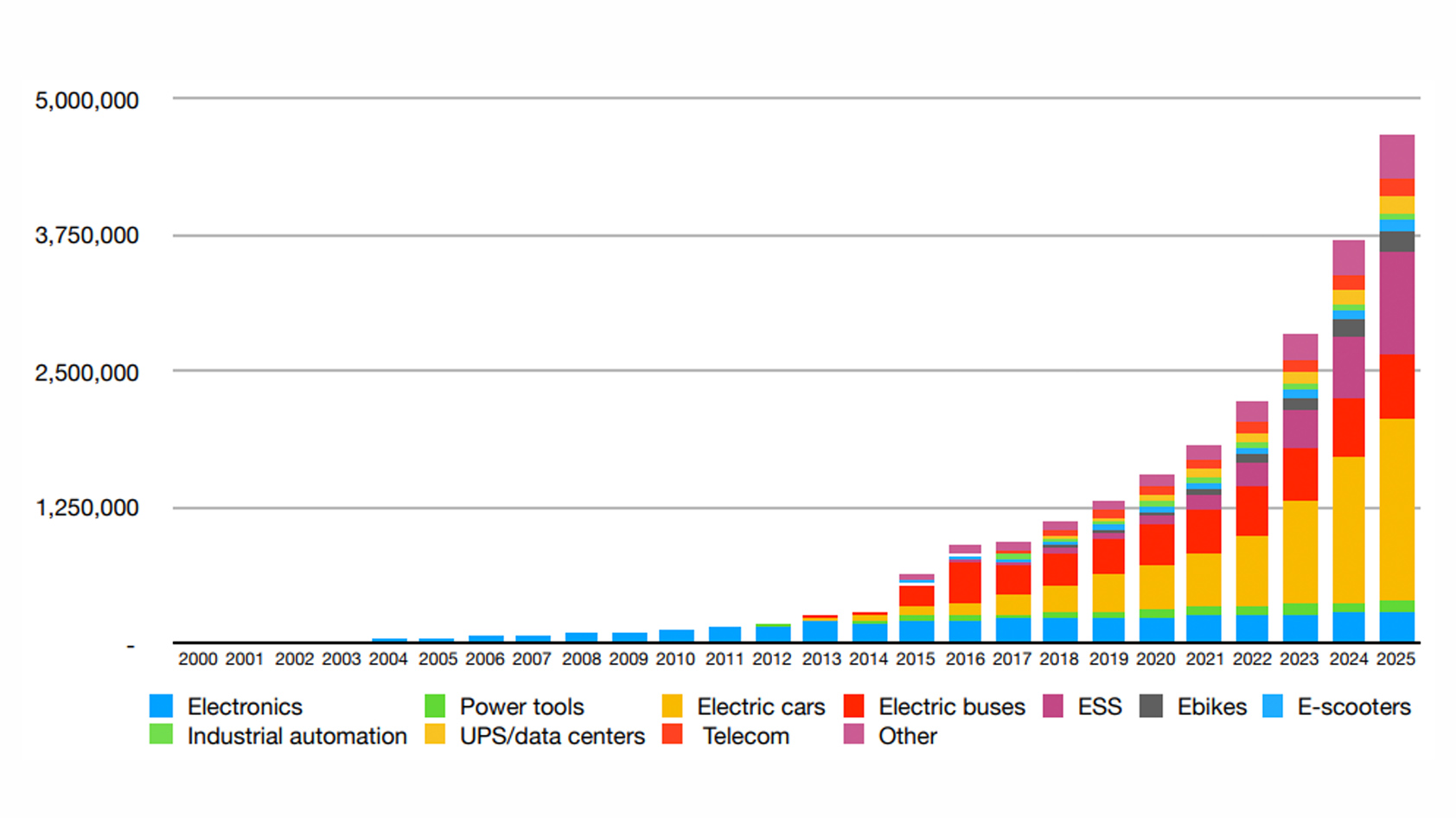
Already a staple in consumer electronics like headphones, lithium-ion batteries also power electric vehicles. Bloomberg New Energy Finance (BNEF) projects that electric cars will make up 34% of sales by 2030, compared to 4% in 2020. This rapid increase in demand translates into upstream adaptations in mining and production.
You may be wondering whether this kind of growth is sustainable and how we’ll deal with all the waste. That’s what we’re here to find out.
Unlike single-use lithium batteries, lithium-ion batteries are rechargeable.
Where do batteries come from?
The Italian physicist Alessandro Volta invented the first true battery in 1800. In 1859, Gaston Planté came up with the first rechargeable battery. Lithium-ion batteries didn’t enter the scene until 1980. And it took 11 more years until they were first commercialized by Sony.
This safe, compact, and energy-dense battery unleashed the mobile revolution, powering camcorders, laptops, smartphones, and most other portable consumer electronics we know today. In 2019, the scientists that invented the lithium-ion battery received the Nobel Prize in chemistry.
Let’s dive into the material makeup of lithium-ion batteries that turned them into these powerful drivers of change.
What are batteries made of?
A battery is a collection of one or more cells. Each electrolyte-filled cell contains two electrodes, each with a current collector, that sit on opposite ends of the battery, with a separator between them. Closing the circuit between the electrodes triggers a series of electrochemical reactions that create an electrical current and discharge the battery. While the basic components and processes are the same in all types of batteries, the materials differ greatly.
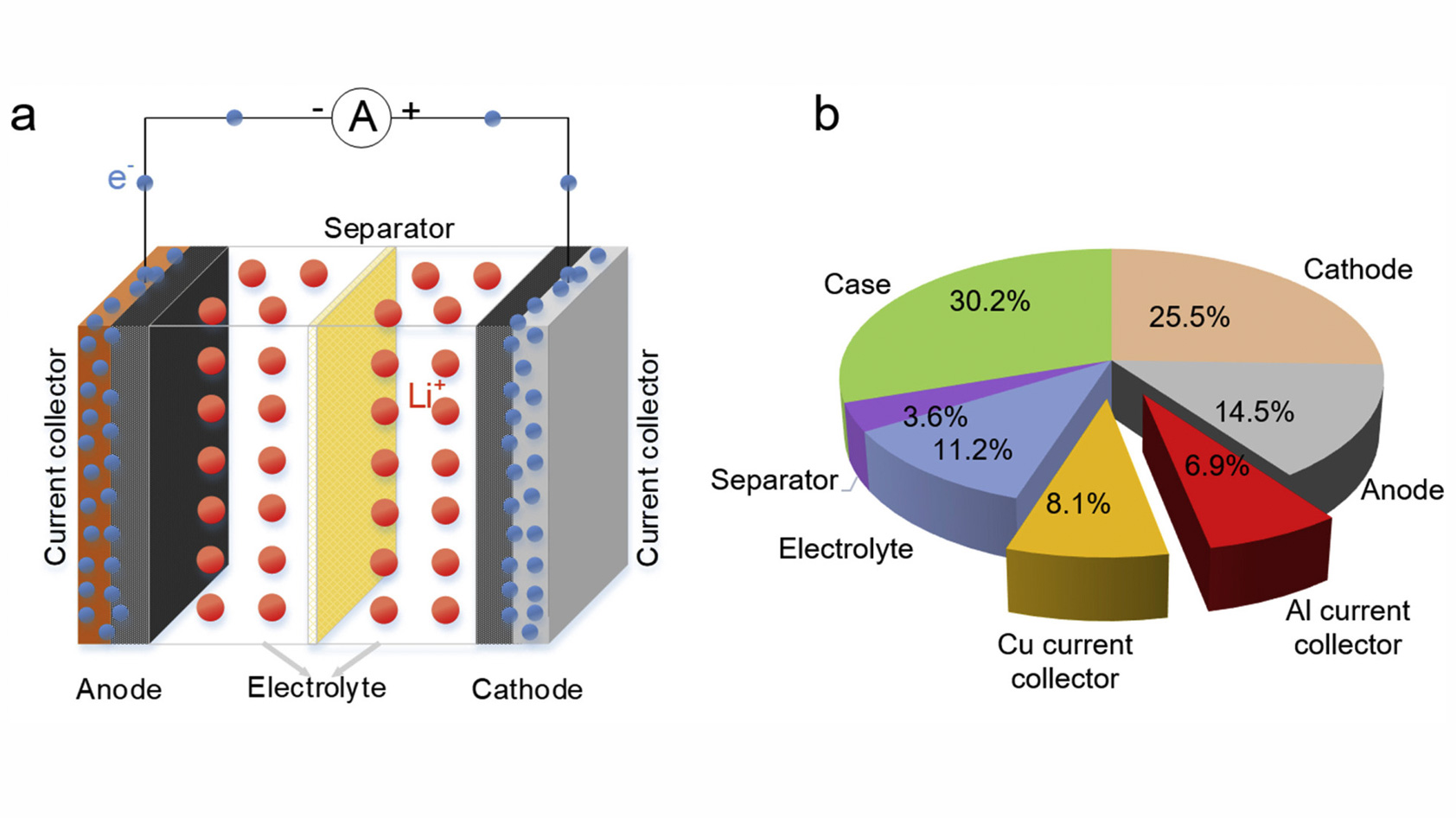
Let’s have a look at the components typically found in a rechargeable lithium-ion battery:
- Anode: lithium stored in carbon structures, more recently in graphite
- Cathode: lithium nickel oxide, lithium cobalt oxide, and/or lithium manganese oxide
- Current collectors: copper, aluminum
- Electrolyte (liquid): lithium salts and organic solvents, generally alkyl carbonates
- Separator: synthetic polymers, specifically polyolefin-based membranes
Where do the materials to make batteries come from?
While most lithium-ion batteries are produced in China, the materials that go into them are scattered across the globe. Here are the most common sources of these materials:
| Material | Natural Reserves | Top Producers (2020) | Extraction |
|---|---|---|---|
| Material Lithium | Natural Reserves Global: 80 million tons Bolivia (26%) Argentina (21%) Chile (12%) Australia (8%) China (6%) | Top Producers (2020) Australia (49%) Chile (22%) China (17%) Argentina (8%) | Extraction Extracted from natural brine in underground lakes (South America) or mineral deposits in hard-rock (Australia). |
| Material Graphite | Natural Reserves Global: 800 million tons Turkey (28%) China (22%) Brazil (22%) Mozambique (8%) | Top Producers (2020) China (62%) Mozambique (11%) Brazil (9%) Turkey (<1%) | Extraction Mining from metamorphic rock. |
| Material Nickel | Natural Reserves Global: 94 million tons Indonesia (22%) Australia (21%) Brazil (17%) Russia (7%) Philippines (5%) | Top Producers (2020) Indonesia (30%) Philippines (13%) Russia (11%) | Extraction Mining from laterites and sulfide deposits. Nickel is also found in manganese crusts and nodules on the ocean floor. |
| Material Cobalt | Natural Reserves Global (terrestrial): 25 million tons Global (ocean floor): 120 million tons Congo (over 50% of terrestrial reserves) Australia (20%) Cuba (7%) Russia (4%) | Top Producers (2020) Congo (68%) Russia (4.5%) Australia (4%) | Extraction Commonly a byproduct of nickel or copper mining. |
| Material Manganese | Natural Reserves | Top Producers (2020) South Africa (28%) Australia (18%) Gabon (15%) Brazil (6%) | Extraction Mined from ore and mainly used in steel production. |
| Material Copper | Natural Reserves Global (identified): 2.1 billion tons Global (undiscovered): ca. 3.5 billion tons Chile (23%) Peru (11%) Australia (10%) China (3%) | Top Producers (2020) Chile (29%) Peru (11%) China (9%) | Extraction Mined globally, including from US mines in Arizona, Utah, New Mexico, Nevada, Montana, Michigan, and Missouri. |
| Material Aluminum (bauxite) | Natural Reserves Global: 55 to 75 billion tons of bauxite Africa (32%) Oceania (23%) South America and Caribbean (21%) Asia (18%) | Top Producers (2020) Australia (30%) Guinea (22%) China (16%) | Extraction Aluminum is a product of alumina smelting, which in turn is produced from bauxite, an ore mined from topsoil. |
All mined minerals undergo refining, often in countries other than their origin.
Mining isn’t the immediate source of the organic solvents and synthetic polymers contained in lithium-ion batteries, although their primary components are extracted from the Earth. Here’s a simplified summary of their production:
- Alkylcarbonates, like diethyl carbonate, are synthesized from phosgene, a gas, and alcohols like ethanol or methanol.
- Polyolefin-based membranes are synthesized from oil- or natural gas-derived polymers.
What are the issues with mining materials?
All mining has social and environmental impacts. Cobalt mining in the Democratic Republic of the Congo, for example, often involves inhumane conditions, as well as slave and child labor. Consequently, manufacturers like Tesla are aiming to use cobalt-free lithium-ion batteries. While mining sources for other minerals may have fewer social impacts, they still require environmental destruction, deplete water resources, and contribute to air, water, and soil pollution.
Mining creates environmental destruction, depletes water resources, and contributes to air, water, and soil pollution.
Material extraction is only the first step. Processing of minerals like lithium usually requires toxic chemicals. Refineries typically dispose of waste in tailings piles or evaporation ponds. From here, poisonous fluids can leak into the environment to contaminate the soil and water. Even processed water may still contain traces of the minerals, which can adversely affect humans and animals.
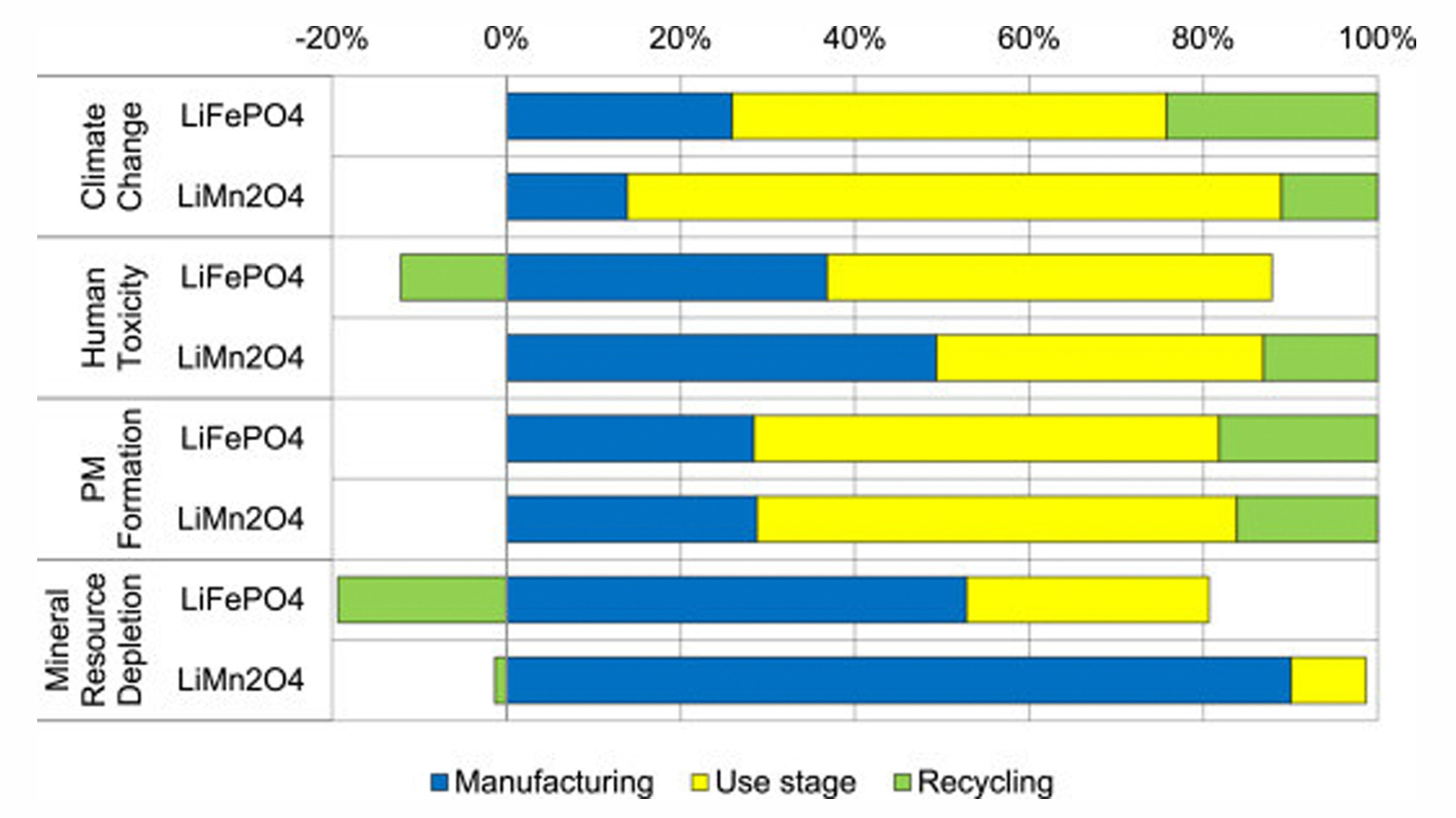
While many materials used in lithium-ion batteries are abundant, they’re not necessarily easy to extract. As natural resources decline, mining operations will have to tackle less favorable sources, which will only increase the negative impacts of extraction and refining and might extend shipping routes. Eventually, resource prices will drive manufacturers to switch to different battery chemistries, for example, from lithium manganese oxide to lithium iron phosphate.
Unfortunately, production isn’t where the trouble ends.
Where do batteries go?
Way too many batteries still end up in a landfill, though it depends on the type. While 90% of lead acid batteries are recycled, experts estimate that only about 5% of lithium-ion batteries currently enter a recycling stream. Many more lurk in drawers or end up in the trash. That’s a problem.
Why you shouldn’t throw batteries in the trash
Lithium-ion batteries can cause fires when exposed to heat, mechanical stress, or other waste materials. Once exposed, the elements contained in the batteries could leach into the environment and contaminate the soil and groundwater. While this shouldn’t present an issue at a well-managed domestic facility, exported trash might end up at a more lenient landfill. Richa et al. note that “the greater risk is loss of valuable materials.”
Sufficiently concentrated natural resources of lithium, cobalt, nickel, and other elements are finite. As discussed above, their mining has irreversible consequences. By the time these materials end up in our gadgets, we’ve paid a high social and environmental price for damage done along their supply chains.
Before long, demand for some materials will exceed mining yields. One recent study projects that lithium and cobalt demand could exceed production as soon as 2025. When you then take into account that, on average, spent lithium-ion battery electrodes contain more Lithium than natural ores, you’ll quickly conclude that even dead batteries have value.
As demand outpaces mining capacities, recycling morphs from an ethical obligation to an economically viable alternative, and possibly a necessity.
Where can consumers safely dispose of batteries?
Batteries are a core component of everyday electronics like smartphones, laptops, or headphones. When the battery dies, this often spells the end of life of the device. That’s particularly true for true wireless earbuds like the AirPods. In many cases, you’ll have to dispose of the entire gadget, rather than just the battery.
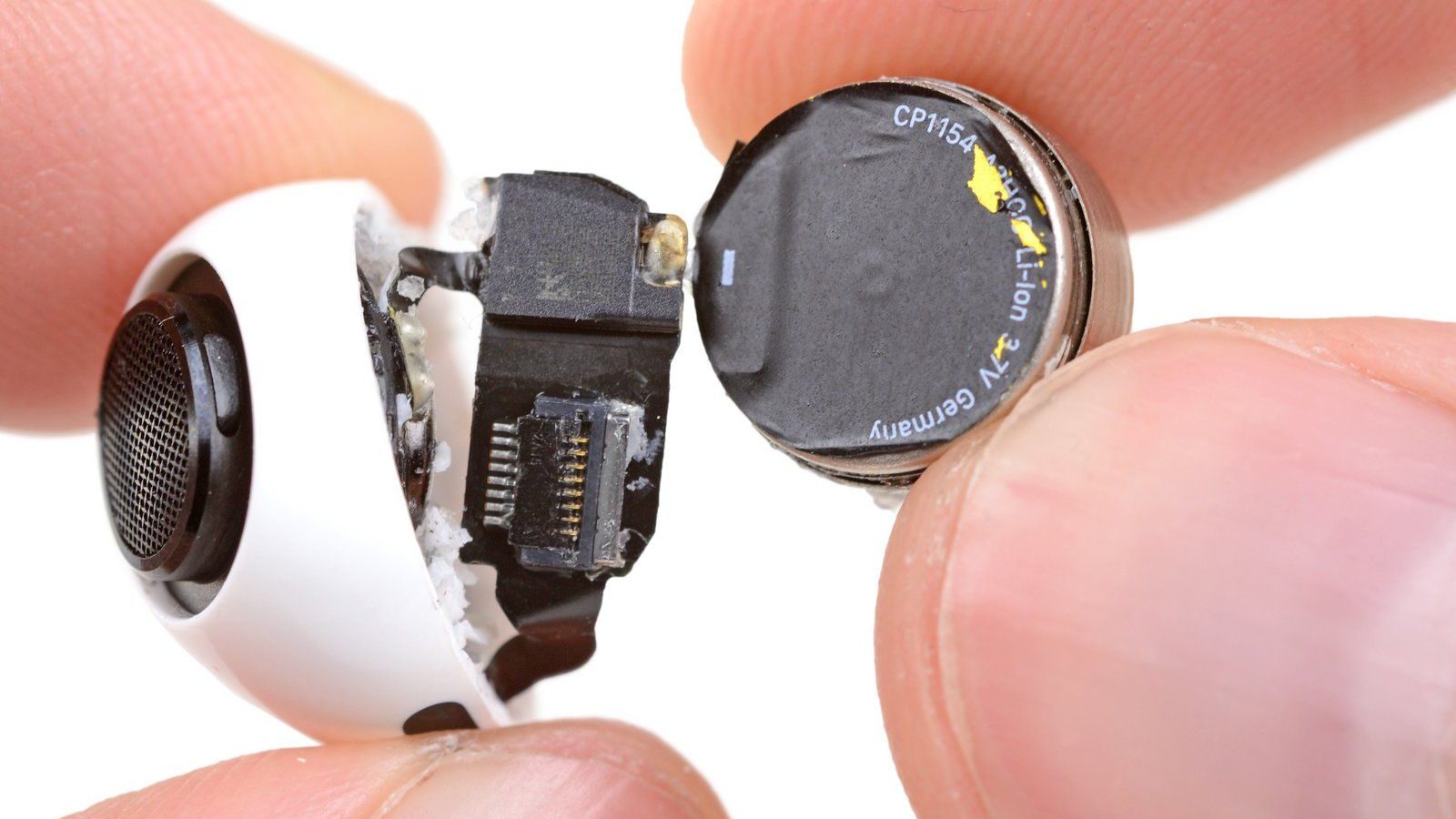
Many manufacturers offer recycling programs for electronic waste. For example, if you have an old iPhone, Apple might trade it in for store credit. Electronics stores like Best Buy will take products back and recycle them for free. If you need to dispose of used household batteries, the EPA recommends searching Earth911 for a local recycling provider. Finally, Call2Recycle offers drop-off locations for batteries and cell phones across the U.S.
Like other electronic devices or batteries, you can find places that take old headphones back or trade them in. In addition to recycling your headphones, you could also try to refurbish, reuse, or sell them. When you’re ready to buy a new pair, consider eco-friendly headphones.
What happens with batteries returned for recycling?
The two most common methods for recycling lithium-ion batteries are pyrometallurgy, a heat-based process, and hydrometallurgy, the leaching of metals with chemicals. Each recycling method has its own set of issues.
Pyrometallurgy is an energy-intense set of operations that produce toxic gases and can recover only some of the elements; lithium and aluminum, for example, are lost in slag, a solid waste byproduct. Hydrometallurgy works at much lower temperatures and has a higher recovery rate, but it’s a much more complex process that uses poisonous chemicals, which create their own waste removal challenge. To maximize resource extraction, the two methods are often used in tandem, but still recover no more than 50% of raw battery materials since they tend to focus on the most valuable metals and neglect others.
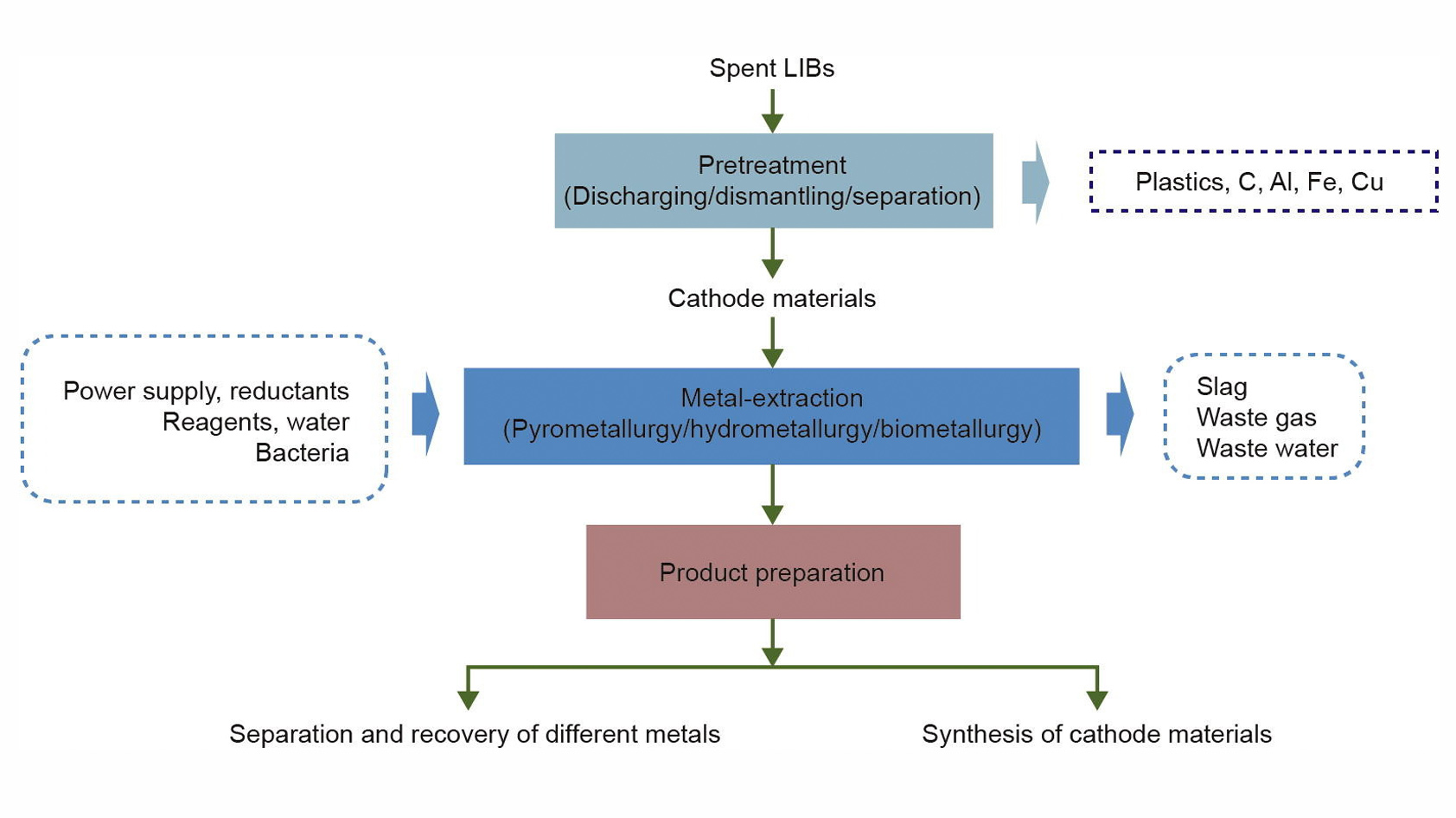
Improved hydrometallurgy-based recycling processes promise to yield recovery rates much closer to 100%. Li-Cycle is one of the first companies to focus exclusively on lithium-ion battery recycling. Its process involves the decentralized disassembly of batteries into their fundamental building blocks, followed by shredding into inert products. From there, materials like plastic, copper, and aluminum go to local recycling streams. The remaining intermediate product, a wet fine powder called black mass, is shipped to a central hub where it’s refined to extract high-value materials like graphite, cobalt, nickel, lithium, and copper. Li-Cycle estimates that it can recover up to 95% of materials with zero diversion to a landfill, no wastewater, and no direct emissions.
Most lithium-ion battery recycling processes focus on valuable materials like the cobalt, manganese, and nickel that make up a lithium-ion battery’s cathode. Unfortunately, lithium is difficult to reclaim, meaning it remains cheaper to mine it. This may change in the coming years, especially if lithium gets more expensive or when more advanced recycling processes become more widely available.
Batteries must enter the circular economy
The production of rechargeable batteries from mined minerals has social and environmental impacts and the natural resources are finite. As demand for this technology continues to increase, both manufacturers and consumers have to step up their recycling game. Manufacturers need to come up with designs that make it easier to remove batteries, disassemble them, and extract individual materials. Meanwhile, consumers should responsibly dispose of spent batteries or old electronics to ensure they enter suitable recycling streams.
By diverting batteries from the landfill, we can recover valuable materials and re-use them for further production. As we increase recycling rates, we’ll lower our dependency on natural resources. That’s the gateway to the circular economy.
Frequently asked questions about batteries
In 2021, Australia produced by far the most lithium (55,000 tons), followed by Chile (26,000 tons) and China (14,000 tons). Interestingly, Bolivia has the largest lithium resource of all (21 million tons), followed by Argentina (19 million tons) and Chile (9.8 million tons). These numbers were taken from the Mineral Commodity Summaries 2022 (PDF), published by the U.S. Department of the Interior and the U.S. Geological Survey.
That’s literally a moving target. The electric vehicle (EV) market, which includes cars, scooters, and bikes, is growing rapidly and so is its environmental impact.
For example, global sales of EV cars more than doubled from 2020 to 2021. With 6.6 million EVs sold in 2021, each containing around 8kg of lithium, the EV market accounts for at least around 58% of the global lithium production (90.7 million kg) in 2021. The social and environmental impacts are the same for all lithium-ion batteries, i.e. habitat destruction, excessive water use, pollution, inhumane mining conditions, etc.
Manufacturers have long feared that batteries made from recycled materials could have a shorter life span or be more prone to battery failures, which could be devastating for an electric car. However, new research published in Joule investigating a novel recycling method for the cathode, an expensive key component in lithium-ion batteries, found that these batteries last longer and charge faster. The lithium-ion battery cathode made from recycled materials is more porous, which keeps the cathode from cracking, a hallmark of lithium-ion battery degradation.
Maybe. While lithium requires mining, just like oil and natural gas, it has a far lower carbon impact. That’s because it’s not actually fuel—we don’t need to burn it and it doesn’t break down into gases that fuel the greenhouse effect. What’s more, once you burn oil or gas, it’s gone. However, it’s possible to recycle lithium and other minerals from spent batteries, meaning they’ll re-enter battery production, which in turn will reduce the need for freshly mined minerals.
On the downside, lithium is toxic and, like oil and gas, its mining potentially pollutes water. The good news is that other, less troublesome, battery technologies may replace lithium-based batteries faster than renewable energy can replace oil and gas.
Out of all the headphones we’ve tested here at SoundGuys, the HyperX Cloud Alpha Wireless last the longest, with an impressive 327 hours and 27 minutes of constant playback on a single charge.